where is the particle before the measurement?
Showing posts with label Quantum physics. Show all posts
Showing posts with label Quantum physics. Show all posts
Wednesday, January 29, 2020
Thursday, July 25, 2019
Bohr's Principle of Complementarity
Bohr's Principle of Complementarity
Bohr was 'one of the intellectual giants of early quantum theory. His ideas and his personality were enormously influential. During the twenties and thirties, the Bohr Institute in Copenhagen (which was financially supported by the Carlsberg Brewery) became a haven for scientists who were developing the new physics. Bohr was not always receptive to quantum ideas; like many of his colleagues, he initially rejected Einstein's photons. But by 1925 the overwhelming experimental evidence that light actually has a dual nature had convinced him. So for the next several years, Bohr concentrated on the logical problem implied by this duality, which he considered the central mystery of the interpretation of quantum theory. Unlike many of his colleagues, Bohr emphasized the mathematical formalism of quantum mechanics. Like de Broglie, he considered it vital to reconcile the apparently contradictory aspects of quanta. Bohr's uneasy marriage of the wave and particle models was the Principle of Complementary. This principle entails two related ideas:
1-A complete description of the observed behavior of microscopic particles requires concepts and properties that are mutually exclusive .
2-The mutually exclusive aspects of quanta do not reveal themselves in the same observations.
The second point was Bohr's answer to the apparent paradox of wave-particle duality: There is no paradox. In a given observation, either quanta behave like waves or like particles.
How, you may wonder, could Bohr get away with this-eliminating a paradox by claiming that it does not exist because it cannot be observed? Well, he has slipped through a logical loophole provided by the limitation of quantum physics that quantum mechanics describes only observed phenomena. From this vantage point, the central question of wave-particle duality is not "can a thing be both a wave and a particle?" Rather, the question is "can a thing be observed behaving like a wave and a particle in the same measurement?" Bohr's answer is no: in a given observation, quantum particles exhibit either wave-like behavior (if we observe their propagation) or particle-like behavior (if we observe their interaction with matter). And, sure enough , no one has yet found an exception to this principle.
Notice that by restricting ourselves to observed phenomena, we are dodging the question, "what is the nature of the reality behind the phenomena?" Many quantum physicists answer, "there is no reality behind phenomena." But that is another story.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
De-Broglie's Law
De-Broglie's Law
If electromagnetic radiation consists of photons-localized clumps of energy-how can we explain phenomena such as diffraction and interference? If not, then why did Compton have to use classical collision theory to explain the scattering of x rays by metals? On the other hand, if electrons are particles, why do they produce an interference pattern at the detector in the double-slit experiment? The behavior of electrons and photons in these experiments seems provocatively similar---crazy, to be sure, but crazy in the same way. Are electrons and photons in some sense the same?
Einstein was deeply puzzled by this question until he noticed a possible answer in the doctoral thesis of a young French physicist. In 1924, Einstein wrote in a letter to his Dutch colleague Hendrik Lorentz (1853-1928) that the research of Prince Louis de Broglie (1892-1975) .....is the first feeble ray of light to illuminate this, the worst of our physical riddles." De Broglie's achievement was to synthesize the wave-like and particle-like aspects of microscopic matter. Although de Broglie seems to have only dimly understood the nature of quantum particles, and his rather nebulous physical models of quanta have since been superseded, the importance of his contribution has not diminished. It initiated the development of modem quantum mechanics.
In 1910 de Broglie began studying history at the University of Paris; soon, however, he switched to physics. His studies were interrupted in 1913 by a six-year stint in the French army, during which he and his brother Maurice worked on wireless telegraphy. Then in 1919 he returned to Paris for his doctoral research. From work on the x-ray spectra of heavy elements, de Broglie knew of photons and the Bohr model of atomic structure. And he was particularly intrigued by "Planck's mysterious quanta." So he set himself the task of "[uniting] the corpuscular and undulatory points of view and thus [penetrating] a bit into the real nature of quanta." In 1923, lightning struck. As de Broglie tells it:
As in my conversations with my brother we always arrived at the conclusion that in the case of x-rays one had [both] waves and corpuscles, thus suddenly-I cannot give the exact date when it happened, but it was certainly in the course of summer 1923-I got the idea that one had to extend this duality to the material particles, especially to electrons.
Thus did de Broglie come up with the idea of matter waves. This idea led him to the important notion that all microscopic material particles are characterized by a wavelength and a frequency, just like photons. Aesthetic considerations seem to have influenced de Broglie's thinking towards the idea of matter waves. He evidently felt that nature should be symmetrical, so if particles of light (photons) were to be associated with electromagnetic radiation, then so should waves of matter be associated with electrons. Simply stated, his hypothesis is this: There is associated with the motion of every material particle a "fictitious wave" that somehow guides the motion of its quantum of energy.
In spite of its rather vague character, this idea was remarkably successful. For example, using the methods of classical optics (such as Fermat's principle) to describe the propagation of quanta, de Broglie was able to explain how photons (and, for that matter, electrons) diffract and interfere: It is not the particles themselves but rather their "guide waves" that diffract and interfere. In de Broglie's words, "the fundamental bond which unites the two great principles of geometrical optics and of dynamics is thus fully brought to light." De Broglie proffered these ideas in his Ph.D. dissertation, which he wrote at age 31. His thesis did not fully convince his examiners, who were impressed but skeptical of the physical reality of de Broglie's matter waves. One examiner later wrote, "at the time of the defense of the thesis, I did not believe in the physical reality of the waves associated with the particles of matter. Rather, I regarded them as very interesting objects of imagination. Nevertheless, de Broglie passed.
De-Broglie's equation
De Broglie's equations for the wavelength and frequency of his matter waves are elegant and simple. Even their derivations are not complicated. In his seminal paper of 1923,
de Broglie began with light quanta-photons-so I'll first recap the derivation of the equation relating the wavelength and momentum of a photon and then press on to material particles.
The photon is a relativistic particle of rest mass mo = O. Hence the momentum p of a photon is related to its total energy E through the speed of light C as
To introduce the frequency v of the photon, we use Einstein's equation for the photon energy
to write Eq. (2.10) as
For a wave in free space, the wavelength is 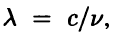 , so Eq. (2.12) becomes
, so Eq. (2.12) becomes
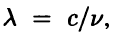 , so Eq. (2.12) becomes
, so Eq. (2.12) becomes
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Wednesday, July 24, 2019
interference experiment of electrons
interference experiment of electrons
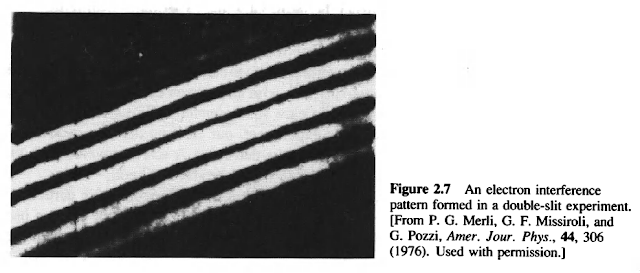
Our strategy in the double-slit experiment is to send electrons through a double-slit diaphragm and see how the intensity measured by the detector differs from the interference pattern formed by light. To implement this strategy, we must make a few modifications in the apparatus Young used when he performed this experiment with light. First we replace the light source with an electron gun-a device that produces a (nearly monoenergetic) beam of electrons of energy E. A heated tungsten wire, for example, produces a stream of electrons that we can accelerate to the desired velocity. Second, we replace the photographic plate with an electron detector: a device that counts the number of electrons that arrive in each square meter of unit area per sec. (Like the photographic plate used in Young's experiment, our electron detector measures the rate at which energy arrives at each point on the detector.) A screen covered with phosphor will do; when an electron arrives at the screen, it produces a spot.
What would we expect to see at the detector if the electrons were particles, subject to the same physical laws as, say, marbles? Imagine for a moment that we block one slit-say, the lower slit in Fig. 2.3-so that all electrons must come through the other, open slit. Most electrons that make it through the diaphragm will go straight through this slit, "piling up" at the detector directly opposite it. We therefore expect to see a maximum in the measured intensity opposite the upper slit. But some particles will scatter from the edges of this slit, so we expect some amount of spread in the pattern. A reasonable guess for the intensity for a beam of particles passing through this apparatus with only the upper slit open is the curve  sketched in Fig. 2.6a. The curve
sketched in Fig. 2.6a. The curve 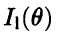 should be obtained if only the lower slit is open.
should be obtained if only the lower slit is open.
 sketched in Fig. 2.6a. The curve
sketched in Fig. 2.6a. The curve 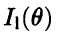 should be obtained if only the lower slit is open.
should be obtained if only the lower slit is open.
What should happen when both slits are open? Well, if the electrons are indeed particles, then the measured intensity should be simply  . This rather featureless curve is sketched in Fig. 2.6b. (Were you to scale the apparatus to macroscopic size and send through it a uniform beam of marbles-with, of course, a suitable detector this is what you would see.) But what we actually see when we run the experiment with electrons is altogether different.
. This rather featureless curve is sketched in Fig. 2.6b. (Were you to scale the apparatus to macroscopic size and send through it a uniform beam of marbles-with, of course, a suitable detector this is what you would see.) But what we actually see when we run the experiment with electrons is altogether different.
 . This rather featureless curve is sketched in Fig. 2.6b. (Were you to scale the apparatus to macroscopic size and send through it a uniform beam of marbles-with, of course, a suitable detector this is what you would see.) But what we actually see when we run the experiment with electrons is altogether different.
. This rather featureless curve is sketched in Fig. 2.6b. (Were you to scale the apparatus to macroscopic size and send through it a uniform beam of marbles-with, of course, a suitable detector this is what you would see.) But what we actually see when we run the experiment with electrons is altogether different.
The measured intensities in Fig. 2.7 clearly exhibit bands of alternating high and low intensity: an interference pattern, like the one formed by light (see Fig. 2.4). This observation seems to imply that the electrons are diffracted by the slits at the diaphragm and then interfere in the region between the diaphragm and the detector. We can even fit the measured intensity of the scattered electrons to the double-slit function  of Eq. (2.6) provided we assign to each electron (of mass m and energy E) a wavelength
of Eq. (2.6) provided we assign to each electron (of mass m and energy E) a wavelength
 of Eq. (2.6) provided we assign to each electron (of mass m and energy E) a wavelength
of Eq. (2.6) provided we assign to each electron (of mass m and energy E) a wavelength
But to a classical physicist, steeped in the idea that electrons are particles, Eq. (2.9) is nonsense!
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Sunday, July 21, 2019
wave-Particle duality(part-3) photons
wave-Particle duality(part-3) photons
Complete planck's work
For his second assault on the energy density of a black body, Planck adopted a statistical method based on the concept of entropy as interpreted probabilistically by Ludvig Boltzmann (1844-1906). At one point in his derivation, Planck introduced a simplifying assumption (i .e., a trick to facilitate his mathematics). His ploy was conventional in mathematical analysis , and Planck expected to remove his assumption at the end. Planck was in for a rude shock.
assumption is both simple and radical. He knew that a classical black body could exchange any amount of energy with the walls of the cavity; nevertheless, for purposes of his derivation, Planck assumed that only discrete amounts of energy can be absorbed or emitted by the resonators that comprise the walls of the black body. He called these discrete amounts of energy "quanta." To each quantum Planck assigned an energy equal to an integral multiple of h*v, where h is the constant h =6.63 X 10^-34 J-sec. Having made this assumption, Planck easily derived the radiation law
where c = 3.0 X 10^8 m-sec is the speed of light and  is Boltzmann's constant. Comparing this result with Eq. (2.1) in wave-Particle duality(part-2) planck's work, we see that in his new equation Planck derived expressions for the constants A and B that appeared in his empirical form.
is Boltzmann's constant. Comparing this result with Eq. (2.1) in wave-Particle duality(part-2) planck's work, we see that in his new equation Planck derived expressions for the constants A and B that appeared in his empirical form.
This derivation was the theoretical justification Planck sought for the distribution of radiation in a black body. But in laying the foundation for his theory, Planck paid an enormous price, for try as he might, he could not get rid of his artificial constant h. Setting h = 0 inevitably led to a result that disagreed with a huge body of experimental data. Yet, if h is non-zero, then Planck's theory is seriously at odds with physicists understanding of energy exchange as a continuous process. Planck's rash assumption heralded the strange new physics of the quantum, which dominated the physics community for the next three decades. But physicists, who were then and are today a rather conservative lot, did not take well to being told that their understanding of so basic a process as energy exchange was fundamentally incorrect. Attempts to derive Planck's result without making his drastic assumption failed, and for several years Planck's quanta languished in obscurity, largely ignored. Yet, Planck's assumption was nothing compared to the surprise Einstein had in store.
photons-Particles of Light
Einstein thought he saw an inconsistency in the way Planck used Maxwell's wave theory of electromagnetic radiation in his derivation. With characteristic intellectual courage, Einstein decided that this inconsistency implied a flaw not in Planck's theory but in Maxwell's. This radical contention shifted the emphasis in research on black bodies from the resonators that comprise the walls to the radiation field itself. Ultimately, it completely altered the way we think about light.
In 1905 Einstein proposed that the energy in an electromagnetic field is not spread out over a spherical wave front, as Maxwell would have it, but instead is localized in indivisible clumps-in quanta. Each quantum of frequency v, Einstein averred, travels through space at the speed of light, C = 3.0 X 10^8 m/sec, carrying a discrete amount of energy h*v and momentum h*v/c. Thus, in Einstein's model, light transports energy in the same way particles do. G. N. Lewis subsequently dubbed Einstein's quantum of radiation energy a photon, the name we use today. The photon model cast Planck's theory of black-body radiation in, so to speak, a new light. Planck thought that energy exchange between the resonators of the cavity and the field occurs in units of h*v because of some strange property of the resonators. To this Einstein said: No, the explanation is that the radiation field itself is quantized. Planck's result is consistent with this extraordinary notion; if the energy in the field is contained in photons-quanta of magnitude h*v-then of course only integral multiples of the photon energy can be exchanged with the walls.
The photon model explained more than just black-body radiation. One of Einstein's greatest achievements was using it to understand the photoelectric effect-the ejection of electrons from a metal, such as sodium, when light impinges on it. (You see the photoelectric effect in action every time an electric eye opens an elevator door for you or closes one on your foot.) Yet, the photon was too radical for physicists of the early 1900's, and Einstein's model of the electromagnetic field encountered strong opposition. Even as late as 1913-years after the publication of Einstein's work on the photoelectric effect-four distinguished German physicists (including Max Planck) wrote in a petition recommending Einstein's appointment to the Prussian Academy of Science:
... That he may sometimes have missed the target in his speculations, as, for example, in his hypothesis of light quanta, cannot really be held too much against him, for it is not possible to introduce fundamentally new ideas, even in the most exact sciences, without occasionally taking a risk.
One year later the American experimentalist R. A. Millikan (1868-1953) reported a precise verification of Einstein's equation for the energy of a photon, E = h*v, and the first measurement of Planck's constant. Yet physicists still resisted abandoning their long-cherished idea that light was a wave. Then, in 1923, Arthur H. Compton (1892-1962) published the results of his x-ray scattering experiments, and drove the last nail into the coffin of the wave theory of light. Compton scattered x-rays-electromagnetic radiation with a wavelength around 10^-10 m and a frequency around 10^18 1/sec- from a thin layer of a light element such as carbon and measured the shift in the wavelength of the x-rays due to the scattering. His results were seriously at odds with the predictions of Maxwell's beleaguered theory. Compton's data clearly showed that the wavelength of the scattered radiation is larger than that of the incident radiation. After several foredoomed attempts to explain this result with classical theory, Compton tried the photon idea. If x-rays carry energy in localized clumps, he reasoned, then we can apply classical collision theory to their interaction with the electrons of the target. Compton used the classical laws of conservation of energy and linear momentum-as though he were analyzing a game of billiards-and was able to derive the correct expression for the wavelength shift. This analysis vindicated Einstein's idea. It was too much even for diehards: the photon was accepted.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
wave-Particle duality(part-2) planck's work
wave-Particle duality(part-2) planck's work
ON THE NATURE OF LIGHT
Corpuscular theories of light, which treated light as though it were composed of particles, have been batting around since the days of Newton and Laplace. But light has an irritating tendency to exhibit distinctively non-particle-like behavior, such as diffraction and interference, which corpuscular theories could not explain. Once Maxwell introduced his wave theory of electromagnetic radiation (in 1870) and it became clear that this theory could beautifully explain such phenomena, most physicists abandoned corpuscular theories of light.
According to Maxwell, all electromagnetic radiation-light included-consists of real waves propagating through space, waves that carry energy distributed over continuous, non-localized spherical wave fronts. In the late 19th century, it seemed that Maxwell's theory could explain even the most complicated electromagnetic phenomena; so convincing were its successes that in 1886, the distinguished physicist Heinrich Hertz (l857-1894)-uncle of Gustav-wrote: "the wave theory of light is, from the point of view of human beings, a certainty." Hertz, as we shall see, was wrong.
the steps of Planck
It was Albert Einstein (1879-1955) who, in 1905, resurrected the notion that electromagnetic radiation is particle-like rather than wave-like in nature . But where did this idea originate? Einstein has written that the seeds of his 1905 theory were planted by research carried out at the turn of the century by the German physicist Max Planck (1858-1947). Although encouraged by his physics teacher to pursue a career as a musician, Planck persevered in physics. His teacher's advice was lousy; Planck's studies of radiation inside a heated cavity led, via a line of reasoning he himself described as an "act of desperation," to the concept of quantization of energy and thence to the birth of quantum physics.
Planck did not set out to revolutionize physics. Instead, following in the footsteps of his teacher G. R. Kirchhoff (1824-1887), he sought to understand why hot bodies glow. This phenomenon, which is called black-body radiation, may be familiar to you if you sculpt. Suppose you have crafted a clay pig. To harden the clay, you fire the pig-i.e., put it in a kiln (an oven) and heat to roughly 2000° F for about 10 to 12 hours. Suppose there is a tiny hole in the oven, too small to admit light but large enough to see through. At first, of course, you see darkness. But as the pig gets hotter and hotter, it begins to glow. As the temperature of the kiln further increases, this glow becomes orange, then yellow, then white, and fills the oven, obliterating all detail of the pig. Why?
Planck formulated this question in slightly more abstract terms, asking: what is the spectrum of electromagnetic radiation inside a heated cavity? More specifically: how does this spectrum depend on the temperature T of the cavity, on its shape, size, and chemical makeup, and on the frequency v of the electromagnetic radiation in it? By the time Planck got into the game, part of the answer was known. Kirchhoff and others had shown that once the radiation in the cavity attains equilibrium with the walls, the energy in the field depends on v and T but, surprisingly, is independent of physical
characteristics of the cavity such as its size, shape, or chemical composition.
The cavity, of course, encompasses a finite volume. Planck was interested in the radiative energy inside the cavity, not on effects that depend on its volume, so he worked in terms of an energy density. In particular, he sought an expression for the radiative energy density per unit volume p(v ,T ). If we multiply this quantity by an infinitesimal element of frequency, we obtain p(v, T )dv, the energy per unit volume in the radiation field with frequencies between v and v + dv at cavity temperature T.
Rather than confront the distracting complexities of a real heated cavity, Planck based his work on a model originally introduced by his mentor Kirchhoff. Kirchhoff called his model of a heated cavity in thermal equilibrium a "black-body radiator." A black body is simply anything that absorbs all radiation incident on it. Thus, a black body radiator neither reflects nor transmits; it just absorbs or emits.
From the work of W. Wien (1864-1928), Planck knew that the radiative energy density p(v, T) for a black body is proportional to v^3 and, from the work of J. Stefan (1835-1893), that the integrated energy density  is proportional to T^4. But this information did not fully describe the dependence of p(v, T) on v and T; experimental evidence implied a further, unknown dependence on v/T.
is proportional to T^4. But this information did not fully describe the dependence of p(v, T) on v and T; experimental evidence implied a further, unknown dependence on v/T.
 is proportional to T^4. But this information did not fully describe the dependence of p(v, T) on v and T; experimental evidence implied a further, unknown dependence on v/T.
is proportional to T^4. But this information did not fully describe the dependence of p(v, T) on v and T; experimental evidence implied a further, unknown dependence on v/T.
Wien had actually proposed an equation for the energy density of a black-body radiator, but the theoretical foundation of his theory was shaky. Moreover, his equation worked only in certain circumstances; it correctly explained the v and T dependence of p(v ,T) for low temperatures and high frequencies. But it predicted that heated black bodies should emit a blue glow at all temperatures-a prediction confounded by our pig. Planck knew of this defect in Wien's theory, for experiments published in 1901 by H. Rubens and F. Kurlbaum conclusively showed it to fail for high temperatures and low frequencies.
In his research, Planck focused on the exchange of energy between the radiation field and the walls. He developed a simple model of this process by imagining that the molecules of the cavity walls are "resonators"-electrical charges undergoing simple harmonic motion. As a consequence of their oscillations, these charges emit electromagnetic radiation at their oscillation frequency, which, at thermal equilibrium, equals the frequency v of the radiation field. According to classical electromagnetic theory, energy exchange between the resonators and the walls is a continuous process-i.e., the oscillators can exchange any amount of energy with the field, provided, of course, that energy is conserved in the process. By judiciously applying classical thermodynamics and a healthy dose of physical intuition to his model, Planck deduced an empirical formula for the radiative energy density:
In this equation, A and B are constants to be determined by fitting to experimental data; that's why it is called "empirical."
Equation (2.1) agreed beautifully with experimental data for a wide range of frequencies and temperatures. And, in the limit v approaches infinity and T approaches 0, it reduced properly to Wien's law. But Planck was concerned that he could not rigorously justify his formula. In a letter dated 1931, he wrote of his dissatisfaction: "a theoretical interpretation had to be supplied, at all costs, no matter how high . . . I was ready to sacrifice every one of my previous convictions about physical laws." Planck had to do just that.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
wave-Particle duality(part-1)
wave-Particle duality(part-1)
The behavior of a microscopic "particle" differs in subtle ways from that of a classical particle; in some respects, it resembles the behavior of a classical wave. So before we plunge into the microworld, let's remind ourselves of the classical pictures of particles and waves.
In Particles and Their Trajectories in Classical Physics we reviewed the characteristics of a classical particle and the analysis of its motion using Newtonian mechanics. For example, a macroscopic particle has a well-defined position and linear momentum at any time, and from these observables we can calculate its energy. Essential to this description is the notion of spatial localization . The idea that a particle is a localized thing is implicit, for example, in the description of transport of energy through space by a particle in a localized lump. This quality of the classical description of a particle is, in turn, reflected in the "state descriptor" of Newtonian theory: the trajectory.
The characteristics of a wave are quite different from those of a particle. A wave is not spatially localized-this quality is reflected in the properties by which we characterize a wave, such as the wavelength . Like a particle, a wave carries energy and momentum, but it does so in a non-localized manner, distributed over a wave front. And a wave exhibits distinctive, non-particle-like behavior such as diffraction, interference, and polarization. Not surprisingly, then, the theory classical physicists use to describe the propagation of a wave and its interaction with matter-the electromagnetic theory of James Clerk Maxwell (l831-1879)-is quite different from that of Newtonian particle dynamics. So at the macroscopic level, classical physics elegantly and cleanly separates into wave mechanics and particle dynamics.
Alas, this division does not apply to microscopic particles, which adamantly refuse to adhere to either the classical wave model or the classical particle model. In some circumstances microscopic particles behave according to the laws of classical mechanics.
For example, some collisions of highly energetic atoms with molecules can be explained by using classical collision theory . Yet, in other circumstances quantum particles behave like waves: e.g., electrons that have been scattered by a crystal exhibit a diffraction pattern when they are detected. Analysis of this pattern reveals that in this experiment the electrons propagate precisely as though they had a well-defined wavelength and frequency . .. as though they are waves.!
"This situation," you may be thinking, "is a mess. You tell me that light isn't a wave. And electrons aren't particles. Well, then, what are they? And how can I understand the behavior of electrons if not with Newtonian mechanics, and of waves if not with Maxwell's theory? What is going on here?"
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Discovery of quantization.
Discovery of quantization in quantum physics.
Quantization is probably familiar to you, but you wouldn't have encountered it in a classical physics book. The observable of macroscopic systems are not quantized. The energy of a ball on a string, the linear momentum of a hockey puck, and the angular momentum of an astronaut in a centrifuge-all can take on any of a continuum of values, subject only to whatever constraints are imposed by forces acting on the system. In the macroscopic world nature is continuous.
Not so in the microworld, where there is abundant evidence that nature is inherently discrete . Physicists have been aware of this fact since pre-quantum days. By 1900, for example, spectroscopes knew that radiation was emitted by atoms and molecules at discrete frequencies. But the first direct evidence of quantization of energy appeared in collision experiments performed in the early 1900's by James Franck (1882-1964) and Gustav Hertz (1887-1975).
For several years, Franck and Hertz had been measuring the ionization potentials of atoms, and this experience influenced their interpretation of the results of their collision experiments.r In these experiments, they scattered electrons from various atoms; for example, in their most famous experiment, Franck and Hertz accelerated electrons emerging from a heated platinum wire, and sent the resulting beam through a gas of mercury vapor. By measuring the current in the scattered electron beam as a function of the energy of the incident electrons, Franck and Hertz could study the energy loss suffered by the electrons in their collisions with atoms of the gas. They found that the scattered current exhibited a series of sharp drops, each occurring at an incident energy equal to an integral multiple of 4.9 e.V. Franck and Hertz were initially led astray by their prior experience studying ionization; they concluded that 4.9 e.V is the ionization potential of mercury. But Neils Bohr,
on learning of Franck and Hertz's results, realized that the electrons were not ionizing the mercury atoms but rather were exciting them-losing energy to the atoms through inelastic collisions. Drawing on his model of the atom, Bohr further deduced that the energy spacing at which the current drops occur, 4.9 e.V, is the separation of two discrete energy levels of the mercury atom.
In 1914, when Franck and Hertz reported their work, physicists thought that it supported the Bohr theory of the atom-which has since been superseded by quantum theory. Nevertheless, the Franck-Hertz experiment stands as one of the first demonstrations of energy quantization in atoms, a striking example of non-classical shenanigans in the microworld.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Friday, July 19, 2019
why is learning quantum mechanics hard?
why is learning quantum mechanics hard?
There are, I think, three reasons.
First, we grew up in and now inhabit a macroscopic world. Our intuition-our sense of how things ought to behave-has been reinforced every waking minute by experiences in a world that abides by classical physics. Moreover, as students of physics, our intuition has been deepened and further reinforced by our study of classical physics. Quantum physics is an affront to that intuition. To understand it, to use it effectively, we must develop a new way of thinking about the way things work, because, as Richard P. Feynman has written:
Things on a very small scale behave like nothing you have any direct experience about. They do not behave like waves, they do not behave like particles, they do not behave like clouds, or billiard balls, or weights on springs, or like anything that you have ever seen.
Second, quantum physics operates at a level that is one step removed from reality. It is more abstract than classical physics. While classical physicists can deal with well defined trajectories that describe particles they can visualize, quantum physicists must forever wander through a haze of uncertainty, probabilities, and indeterminacy, trying to understand a universe they cannot directly observe. The microcosm can be understood, but it cannot be seen.
Third, quantum mechanics is inherently mathematical. There is an important distinction here between classical and quantum physics. In classical physics we use mathematical methods to implement the ideas of the theory, but we can discuss those ideas without recourse to mathematics. This is not possible with quantum mechanics. Notwithstanding the achievements of writers who have recently tried to popularize quantum theory-several of whose books believe it is impossible to fully grasp the principles of quantum theory without seeing them expressed mathematically. Math is more than a tool for solving quantum mechanics problems: mathematics is the language of quantum physics. As we move deeper into quantum theory, we'll see physics and math become inextricably intertwined. Thus quantum physics demands that we think in a new, abstract, inherently mathematical way-no easy task.
Finally, we must re-think and redefine many familiar words and concepts, and when we use these words to discuss quantum concepts, we must do so with great care and precision. Familiar terms such as "particle" and "motion" assume in the quantum domain subtle overtones and shades of meaning that we ignore at our peril. The "position" of a quantum particle prior to its measurement is not a single, well-defined number, such as IO.7m. Rather it must be specified as a collection of several-maybe an infinity of-values, none of which represent the position of the particle. Instead, each value represents a possibility, a location at which the particle might be found.
represents a possibility, a location at which the particle might be found. In spite of these roadblocks-our classical intuition, the inherently abstract, mathematical nature of quantum mechanics, and the need to use old words in new ways-I believe that you can understand quantum physics. Even if you cannot visualize what goes on in the micro world of atoms and molecules, you can grasp the beautiful and powerful physical laws that govern that world. After all, countless physicists have done it.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Thursday, July 18, 2019
The Indeterminate Universe
The Indeterminate Universe
As this whirlwind overview may suggest, the new physics, quantum physics, differs from classical physics in nearly every way imaginable-from its elements and laws to the kind of knowledge it provides. It is in this last respect that the implications of the quantum revolution are most profound.
Classical physics yields precise information about the properties and behavior of individual, independent systems. It holds out to the scientist the promise that, in principle at least, he can know everything. Quantum physics says that this is just not so, that nature imposes fundamental limitations on knowledge, constraints such as the Heisenberg Uncertainty Principle.
These limitations derive from the probabilistic nature of quantum mechanics. The new physics yields only statistical information about aggregates of identical systems. It is mute about the behavior of individual systems. Moreover, the statistical information provided by quantum theory is limited to the results of measurements. That is, quantum physicists are not allowed to infer facts about a system unless these facts could be verified by experiment. This is a severe limitation-for example, it prohibits us from ascribing an orbit (a path) to a particle, because measurements of position are necessarily performed at discrete times. Even if we measure the position of a particle at two very nearby times, t1 and t2, we cannot even make the simple inference that it traveled from r(tt} to r(t2) via a path in geometric space; we can only say that we found it at r(t1) at tl and then at r(t2) at t2.
This limitation, that physics describes only observed phenomena, forces us to think about reality in a strange, new way. At its most fundamental level, reality seems to be discontinuous. Here is Erwin Schrodinger, the father of quantum mechanics, on this most non-classical feature of his theory:
it is better to regard a particle not as a permanent entity but as an instantaneous event. Sometimes these events form chains that give the illusion of permanent beings-but only in particular circumstance and only for an extremely short period of time in every single case.
In closing, I should note that alI this has not set welI with many physicists. Even today, some find the world view implied by quantum mechanics-a subjective universe, acausal and indeterminate, where the knowledge accessible to man is limited-to be intolerably offensive. And the battle over the interpretation of quantum mechanics goes on. But all agree on the usefulness of the theory for predicting and understanding the physics of matter. It is, after all, the only game in town.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Relation between Classical and Quantum Physics
Relation between Classical and Quantum Physics
To say that classical physics died around 1930 is a bit too
dramatic. The physics of Newton and Maxwell still accurately and beautifully
describes the macroscopic world. Knowing this, physicists developing quantum
mechanics demanded that when applied to macroscopic systems, the new physics
must reduce to the old physics. Thus, as the size of the system being studied
increases, the quantum laws of motion must go over smoothly into those of
Newtonian mechanics, and non-classical phenomena such as uncertainty and
duality must become undetectable. Neils Bohr codified this requirement into his
Correspondence Principle, the essential elements of which are sketched in Fig.
1.3. We shall return to this principle repeatedly, since the ability of quantum
mechanics to satisfy it is an important theoretical justification of the new
physics.
The experimental justification of quantum theory is that it
works. The predictions, qualitative and quantitative, of quantum mechanics have
been verified by a host of experiments on a diverse collection of systems.
These verifications are of paramount importance to physics, for in some ways
quantum theory is a fragile creation. It is an inferential theory, in which we
devise a handful of postulates and from them derive equations, which can then
be put to the test of experimental justification. If the equations pass this
test, then we have confidence that the postulates, which cannot be directly
tested, are correct. But it would take only one repeatable experiment whose results
confounded the equations of quantum mechanics to bring the whole structure tumbling
down. To date, the edifice stands. Quantum mechanics remains the only theory we
have to explain how matter is constructed and how physical processes at the
atomic level work.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
The Fall of the Trajectory to uncertainty principle
The Fall of the Trajectory to uncertainty principle
So much for the idea of using the classical model of a particle to describe a microscopic entity. But can we salvage the basic state descriptor of classical physics, the trajectory? The first step in determining the trajectory of a particle is measuring its initial conditions, x (t0) and Px(t0). To determine the accuracy of our results, we would perform such a measurement not on just one particle, but on a large number of identical particles, all in the same state. Each individual measurement yields a value for x and a value for Px (subject to experimental uncertainties). But the results of different measurements are not the same, even though the systems are identical. If graphed, these results are seen to fluctuate about a central peak, as illustrated in Fig. 1.2. (A similar spread of results characterizes measurement of the y- or z- components of position and momentum.) At first, the fact that the results of many identical experiments are not the same doesn't worry us; we think it's just a manifestation of the experimental error that bedevils
all measurements. According to classical physics, we can
reduce the errors in x and Px to zero and thereby determine precisely the
initial conditions. But we cannot unambiguously specify the values of these
observables for a microscopic particle. This peculiarity of nature in the
microscopic realm is mirrored in the Heisenberg Uncertainty Principle (HUP). In
its simplest form, the HUP shows that any attempt to simultaneously measure
x(to) and Px(to) necessarily introduces an imprecision in each observable. No
matter how the experiment is designed, the results are inevitably uncertain,
and the uncertainties.
, which are measures of fluctuations like those in Fig. 1.2,
cannot be reduced to zero. Instead, their product must satisfy the condition.
where h is Planck's Constant
Not a very big number, but not zero either. [Similar
constraints apply to the pairs of uncertainties.

.] Position and momentum are fundamentally incompatible
observables, in the sense that knowing the precise value of one precludes
knowing anything about the other. But there is a deeper dimension to the
Heisenberg Uncertainty Principle. Quantum theory reveals that the limitation
reflected by Eq. (1.4) on our ability to simultaneously measure x and Px is
implicit in nature. It has nothing to do with a particular apparatus or with
experimental technique. Quantum mechanics proves that a particle cannot
simultaneously have a precise value of x and a precise value of Px.
Similar uncertainty principles constrain our ability to
measure other pairs of incompatible observables. But uncertainty relations such
as (1.4) are not veiled criticisms of experimental physicists. They are
fundamental limitations on knowledge: the universe is inherently uncertain. Man
cannot know all of existence. We might think of uncertainty relations as
nature's way of restraining our ambitions.
The Heisenberg Uncertainty Principle strikes at the very
heart of classical physics: the trajectory. Obviously, if we cannot know the
position and momentum of a particle at t0 we cannot specify the initial
conditions of the particle and hence cannot calculate its trajectory. Indeed,
since the HUP applies at any time, it makes no sense to ascribe a trajectory to
a microscopic particle. We are forced, however reluctantly, to conclude that
microscopic particles do not have trajectories. But once we throw out
trajectories, we must also jettison Newton's Laws. And there goes the ball
game: stripped of its basic elements and fundamental laws, the whole structure
of classical physics collapses.
The demise of classical physics occurred around 1930.
Sitting amidst the rubble, physicists of that era realized that their only
alternative (other than to change careers) was to rebuild-to construct from
scratch a new physical theory, one based on elements other than trajectories
and on laws other than those of Newton and Maxwell. Thus, began the quantum
revolution, whose reverberations are still being felt.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Wednesday, July 17, 2019
wave-particle duality (When Is a Particle Not a Particle?)
wave-particle duality (When Is a Particle Not a Particle?)
In describing the quantum world, we must abandon the
classical notion of a particle as
an indivisible mass point with an independent existence and
well-defined, measurable
extrinsic properties. Whatever microscopic entities are, they are certainly
not indivisible mass
points. We can verify this assertion in the laboratory. In suitably designed
experiments quantum
"particles" act like classical particles-appearing, for example, as
spots on a screen. But
in other experiments their transit through space is like that of a wave, manifesting behavior such as
diffraction and interference, as though they were diffuse wave fronts. This apparently contradictory behavior is a
manifestation of the wave-particle
duality that characterizes the domain where quanta dwell.
we'll see why it poses such a challenge to the student of quantum physics: it renders useless the
conventional mental images with
which we visualize physical phenomena. Thus, an atomic electron is not a
tiny "planet" orbiting
a "nuclear sun," as in the Rutherford and Bohr models of the atom.
But neither is
it a "fuzzy" thing, smeared out over a region of space, as it is
portrayed in many introductory
physics texts. The electron is something else, neither particle nor wave but eerily reminiscent of both.
The dual nature of subatomic particles subverts the
classical concept of a particle. But the true nature of microscopic entities is
even more nebulous than is implied by wave-particle duality, for the properties
of quantum particles are not, in general, well defined until they are measured.
In a sense, the physical properties of electrons, protons, and the like are
"potential" or valent properties until an experimenter-a macroscopic
being-performs a measurement.
You will encounter this disquieting aspect of quantum
mechanics if you ask a quantum physicist to predict the value you would obtain
were you to measure, say, the position of an electron in a metal. He cannot
give you a definite answer, even if he knows fully the state of the electron just
prior to the proposed measurement. The inability of quantum theory to provide
precise answers to such simple questions is not a deficiency of the theory;
rather it is a reflection of its essential nature. We can see this if we look
at how quantum mechanics specifies the state of a particle.
Unlike a classical state, a quantum state is a
conglomeration of several possible outcomes of measurement of physical
properties. At most, quantum physicists can tell you only the possible outcomes
and the probability that you will obtain one or another of them. Quantum
mechanics is expressed in the language of probabilities, not certainties. It is
inherently statistical in nature, describing not definite results of a
measurement on an individual system, but rather possible results of
measurements on a large number of identical systems. What, then, controls what
actually happens in a particular measurement--e.g., which of the possible
values of position a particle exhibits when we measure this quantity? Random chance.
Of course, were we to carry out a position measurement on a
single particle, we would get a single value. So immediately after the
measurement, we can meaningfully talk about the position of the particle-its
position is the number we got in the measurement. But what about immediately
before the measurement? According to quantum mechanics, the particle does not
then have a position. Rather, its position prior to measurement is latent-a
mere possibility, waiting to be made actual.
Thus, by the act of measurement, we change the state of the
particle from one in which it is characterized by a plethora of possible
positions to one in which it has a single, well-defined position. Clearly,
measurement will play a more vital role in quantum physics than in classical
physics. When we study the microworld, experimentation is not just a way of
discovering the nature of external reality, but rather is a way of creating
certain aspects of reality! In contrast to the assumptions of classical
physics, an observer cannot observe a microscopic system without altering some
of its properties.
Intrinsically indeterminate interactions between the
observer and the observed are an inevitable feature of the quantum universe,
one that applies not just to position, but to all properties of microscopic
particles. Some physicists believe that we macroscopic observers
"create" the microscopic building blocks of the universe by
actualizing via measurement their various physical properties.
This interaction is unavoidable: the effect of the observer
on the observed cannot be reduced to zero, in principle or in practice. This
fact, which is reflected in the mathematical structure of quantum theory and
has been verified by countless experiments, demolishes the philosophical notion
of an objective universe, the idea that what we study in physics is necessarily
a "real world" external to and independent of our perceptions.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
determinism of universe
determinism of universe
The underlying assumptions and philosophical implications of
classical physics are so familiar that you may have never given them a second
thought. For example, classical physics ascribes to the universe an objective
reality, an existence external to and independent of human observers. This
assumption reassures us that when we do research in physics, we are studying
what is actually "out there," beyond and distinct from our
consciousness, and that we can design experiments that do not affect in any significant
way the systems they measure. That is, in an objective universe we can control
the interaction between the observer and the observed and, if clever enough,
can make this interaction negligibly weak.
Our other central assumption about the nature of the
classical universe is that it's predictable: knowing the initial conditions of
the constituents of any system, however complicated, we can use Newton's Laws
to predict its future. Precisely, without ambiguity or uncertainty. This notion
is the essence of determinism, which, supported by Newtonian mechanics,
dominated philosophical thinking until the advent of quantum theory. If the
universe is determinate, then for every effect there must be a cause. After
all, if all events, all phenomena can be predicted and explained precisely by
physics, then it must be possible to backtrack from any event to find,
somewhere in the dim recesses of history, its cause. We may have to look long
and hard-the bird that just fell off a tree limb outside my window may have
been influenced by an event during the Peloponnesian wars-but somewhere there
is a cause. Or so says the principle of causality.
Causality was an important element of nineteenth century
philosophy. It had important implications for physicists of that era, for it
guaranteed the reproducibility of experiments-i.e., that two identical systems
with the same initial conditions (i.e., in the same state) subject to the same
measurement will yield identical results. Same causes: same effects. Very neat
and tidy.
I suppose the deterministic world view implied by Newtonian
mechanics could, even today, seem comforting, for it implies that the universe
can be understood fully and dealt with rationally. But it has a dark side. For
example, if the universe is ruled by causality, then free will is a meaningless
concept, for the universe is a vast, mindless machine that controls our every
action, our every decision. Everything that happens happens because of
something that happened in the past, not because we chose to make it happen.
According to this view, if you "decide" to get married or to take a
job or to collect water buffalo, you do so not by choice but because of past
events. In such a universe, our loves, hopes, and dreams are but delusions,
hiding the grim reality that we are but cogs in Descartes' clockwork machine.
Goals are irrelevant; human aspirations pointless.
Thus, determinism is a dehumanizing philosophy. It describes
a universe that is infinitely predictable, holding few surprises. All can be
known; nothing is unexpected.
Determinism is boring. It is also wrong.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek
Tuesday, July 16, 2019
Why Quantum Physics
Why Quantum Physics
How does the universe work? What are the principles that underlie natural phenomena? Philosophers have pondered such questions for
millennia; their answers reflect the world view of Western civilization-how we
perceive ourselves in relation to the universe. In the early years of this
century the dominant world view was determinism, a tenet that had its origins
in the philosophical musings of Descartes. Descartes' metaphor for the universe
was a gigantic clockwork machine, a Rube Goldberg device that grinds
relentlessly onward, towards eternity. According to this interpretation, the
future is preordained and fully predictable. All of existence is described
precisely by physical laws, and, in principle, can be known by man. Nature,
however, had a surprise in store for philosophers. In the first decades of the
twentieth century, they were to see this world view subverted, uprooted, and
toppled by a young upstart physical theory called quantum mechanics.
Theoretical physics has come a long way since those fateful days in the 1920's
and 30's when physicists were taking the first tentative steps towards a
radical new definition of reality. So far have we come that in 1981 the
physicist Steven Hawking could reasonably predict that
... by the end of the century. ... we might have a complete,
consistent, and unified theory of the physical interactions which would
describe all possible observations.
The foundation of that unified theory is quantum mechanics,
the subject matter of this book. What is quantum mechanics? You probably know
that if you want to understand how a transistor works or the principles behind
the laser in your compact disc player you'd better not use classical physics.
Newton 's mechanics and Maxwell's electromagnetic theory can explain macroscopic
phenomena, such as the motion of billiard balls or rockets, but fail
spectacularly when applied to microscopic phenomena, such as proton-atom
scattering or the flow of electrons in a semiconductor. An understanding of
such processes requires a better mousetrap: quantum mechanics. More precisely,
quantum mechanics is a collection of postulates based on a huge number of
experimental observations, and the tools derived from those postulates. We use
these tools to analyze, predict, and understand microscopic phenomena. Hence
quantum theory forms the bedrock of the modem physics of matter-atoms,
molecules and solids-and is at the core of such diverse sciences as
astrophysics and biochemistry. In its more pragmatic guises, quantum mechanics
plays a vital role in a vast array of technological applications, some of which
we use every day-e.g., the microchips in the computers.
The source:
Michael A. Morrison - Understanding Quantum Physics.
By. Fady Tarek